DIFFERENCE BETWEEN DIFFUSION AND OSMOSIS
DIFFUSION
The movement of substances or molecules, atoms, ions from the region of higher concentration to the region of lower concentration until molecules are evenly distributed, is called diffusion.
OSMOSIS
Osmosis is the movement of a solvent across a semipermeable membrane from a region of lower concentration of solution toward a higher concentration of solution.
It is specific type of diffusion in which diffusion occurs across a semipermeable membrane (SPM) and only the water or other solvent moves.
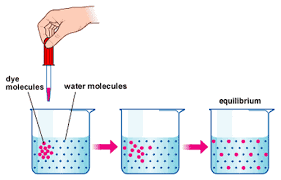
SIMILARITIES & DIFFERENCES
PARTICULARS | DIFFUSION | OSMOSIS |
Similarities | Works as passive transport system and don’t need additional energy to function | Works as passive transport system and don’t need additional energy to function |
Equalize the concentration of two solution | Equalize the concentration of two solution | |
Differences | Don’t require a semi-permeable membrane | Always require a semi-permeable membrane |
Both solvent and solute particles are free to move in diffusion | In biology, it occurs in liquid and Only solvent (water) molecules passes the membrane | |
The flow of particles occur in all direction | Occurs only in one direction. | |
Diffusion can occur in any medium, whether it is liquid, solid, or gas | Osmosis occurs only in a liquid medium | |
Mainly depends on the presence of other particles, and is not depend on solute potential and/or water potential | Osmosis depends on solute potential means number of solute particles dissolved in solvent |
Read more..
WHAT OSMOSIS IS- DEFINITION & FACTS
WHAT DIFFUSION IS-FACTS,ROLE&IMPORTANCE
CLASSIFICATION OF ENZYMES