HYDROLYSIS- REACTION & OCCURRENCE
INTRODUCTION
Hydrolysis refers to any chemical reaction in which a molecule of water breaks one or more chemical bonds.
Usually it is a chemical process in which a molecule of water is added to a substance. Sometimes this addition causes both substance and water molecule to split into two parts. In such reactions, one fragment of the parent molecule gains a hydrogen ion. It breaks a chemical bond in the compound.
- The term Hydrolysis is derived from Greek hydro means ‘water’, and lysis means ‘to unbind or break’ and is used in reactions in which water acts as nucleophile (A nucleophile is a chemical substance that donates an electron pair to form a chemical bond in relation to a reaction.)
- Hydrolysis is usually associated with surface waters but also takes place in the atmosphere, groundwater, at the particle–water interface of soils and sediments, and also in living organisms.
- More specifically, hydrolysis is a double decomposition reaction with water as one of the reactants. For example, if a chemical is represented by the formula AB in which A and B are atoms or groups and water is represented by the formula HOH, the hydrolysis reaction is represented by the reversible chemical equation:
AB + HOH ⇌ AH + BOH
- Hydrolysis involves the reaction of an organic chemical with water to form two or more new substances and usually means the cleavage of chemical bonds by the addition of water.
- Hydrolysis can be the reverse of a condensation reaction in which two molecules join together into a larger one and eject a water molecule. Therefore, hydrolysis adds water to break down, whereas condensation builds up by removing water.
- Typically in a hydrolysis reaction, the hydroxyl group (OH−) replaces another chemical group in the molecule and hydrolysis reactions are usually catalyzed by hydrogen ions or hydroxyl ions.
OCCURRENCE
- Salt
A common kind of hydrolysis occurs when a salt of a weak acid or weak base (or both) is dissolved in water. The water spontaneously ionizes into hydroxide anions and hydronium cations.
For example, the salt using sodium acetate (CH3COONa) dissociates into the constituent cations (Na+) and anions (CH3COO−). The sodium ions tend to remain in the ionic form (Na+) and react very little with the hydroxide ions (OH−) whereas the acetate ions combine with hydronium ions to produce acetic acid (CH3COOH).
- Esters and amides
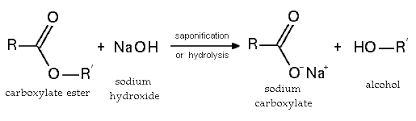
Acid–base-catalysed hydrolyses are very common; for instance the hydrolysis of amides or esters. Their hydrolysis occurs when the nucleophile (water or hydroxyl ion) attacks the carbon of the carbonyl group of the ester or amide. The formation of soap (saponification) is common example of ester hydrolysis. It is the hydrolysis of a triglyceride (fat) with sodium hydroxide (NaOH). During the process, glycerol is formed, and the fatty acids react with the base, converting them to salts. These salts are called soaps, which is common in households uses.
In living systems, most biochemical reactions (including ATP hydrolysis) take place during the catalysis of enzymes. The catalytic action of enzymes allows the hydrolysis of proteins, fats, oils, and carbohydrates.
The hydrolysis of peptides gives amino acids.
- ATP (Adenosine Triphosphate)
Hydrolysis is related to energy metabolism and storage. The energy derived from the oxidation of nutrients is not used directly but, by means of a complex and long sequence of reactions, it is channelled into a special energy-storage molecule, called ATP. The ATP molecule contains pyrophosphate linkages (bonds formed when two phosphate units are combined together) that release energy when needed. ATP can undergo hydrolysis in two ways: Firstly, the removal of terminal phosphate to form adenosine diphosphate (ADP) and inorganic phosphate, with the reaction:
ATP + H2O → ADP + Pi
Secondly, the removal of a terminal diphosphate to yield adenosine monophosphate (AMP) and pyrophosphate.
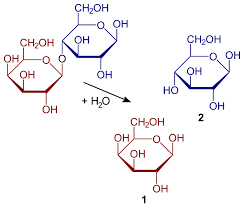
- Polysaccharides
Monosaccharides can be linked together by glycosidic bonds, which can be cleaved by hydrolysis. Two, three, several or many monosaccharides thus linked form disaccharides, trisaccharides, oligosaccharides, or polysaccharides, respectively. Enzymes that hydrolyse glycosidic bonds are called “glycoside hydrolases” or “glycosidases”.
Hydrolysis of sucrose yields glucose and fructose. Invertase is a sucrase which is used industrially for the hydrolysis of sucrose to so-called invert sugar.
Lactase is essential for digestive hydrolysis of lactose in milk; many adult humans do not produce lactase and cannot digest the lactose in milk.
The hydrolysis of polysaccharides to soluble sugars can be recognized as saccharification (any chemical change wherein a monosaccharide molecule remains intact after becoming unbound to another saccharide that it was attached to.)