SOIL COLLOIDS- PROPERTIES, TYPES AND IMPORTANCE
Soil Colloids
- Soil colloids are the finer size fractions (clay and organic matter) of the soil. Because of their large surface area and the chemical structure of the materials involved, soil colloids are also considered as the most chemically active portion of the soil.
- The clay fraction of the soil contains particles less than 0.002 mm in size. Particles less than 0.001 mm diameter in size possess colloidal properties known as soil colloids.
- The colloidal state is a two-phase system in which one material in a very finely divided state is dispersed through second phase.
For examples– solid in liquid -Dispersion of clay in water and
Liquid in gas (Fog or clouds in atmosphere). - Size– The inorganic and organic colloids are extremely small in size (< 0.001 mm in diameter). These particles can be seen only with an electron microscope.
- Surface area- All soil colloids have a larger external surface area per unit mass due to their small size. The external surface area of 1 g of colloidal clay is 1000 times that of 1 g of coarse sand. The total surface area of soil colloids ranges from 10 m2 /g for clays with only external surfaces to more than 800 m2 /g for clays with extensive internal surfaces.
- Surface charges– Both external and internal surfaces of soil colloids carry negative and/or positive charges. Most of the organic and inorganic soil colloids carry a negative charge. When an electric current is passed through a suspension of soil colloidal particles they migrate to anode, the positive electrode indicating that they carry a negative charge. The magnitude of the charge is known as zeta potential. The presence and intensity of the particle charge influence the attraction and repulsion of the particles towards each other, there by influencing both physical and chemical properties.
The sources of negative charge on clays come from ionizable hydrogen ions and isomorphous substitution.
Ionizable hydrogen ions: These are hydrogen from hydroxyl (OH) ions on clay surfaces. The -Al-OH or -Si-OH portion of the clay ionizes the H and leaves an unneutralized negative charge on the oxygen (-AlO or – SiO-). More ionization occurs in alkaline (basic) solutions.
Isomorphous substitution: This is due to the substitution of a cation of higher valence with another cation of lower valence but similar size in the clay crystal structure. In clay crystals some ions fit exactly into mineral lattice sites because of their convenient size and charge. Dominantly, clays have Si4+ in tetrahedral sites and Al3+ in octahedral sites. Other ions present in large amounts during clay crystallization can replace some of the Al3+ and Si4+ Common substitutions are the Si4+ replaced by Al3+, and replacement of Al3++ by Fe3+, Fe2+, Mg2+ or Zn2+. As the total negative charge from the anions (oxygen) remains unchanged, the lower positive charge of the substituted cations result in excess negative charges on clay crystals. - Adsorption of cations- As soil colloids possess negative charge, they attract ions of positive charge like H+, Al3+, Ca2+ and Mg2+ on the colloidal surfaces. This gives rise to an ionic double layer.
- Adsorption of water– A large number of water molecules are associated with soil colloidal particles. Some water molecules are attracted to the adsorbed cations and others water molecules are held in the internal surfaces of the colloidal clay particles. These water molecules play a critical role in determining both the physical and chemical properties of soil.
- Cohesion & Adhesion – The tendency of clay particles to stick together is called cohesion. This tendency is due to the attraction of clay particles for water molecules held between them. When colloidal substances are wetted, water first adheres to individual clay particles and then brings about cohesion between two or more adjacent colloidal particles.
Adhesion refers to the attraction of colloidal materials to the surface of any other substance with which it comes in contact. - Swelling and shrinkage- Some soil clay colloids from smectite group like Montmorillonite swell when wet and shrink when dry. After a prolonged dry spell, soils high in smectite clay (e.g. Black soil -Vertisols) often show crises-cross wide and deep cracks. These cracks first allow rain to penetrate rapidly. Later, because of swelling, the cracks will close and become impervious.
But soils dominated by kaolinite, chlorite, or fine grained micas do not swell or shrink. Vermiculite is intermediate in its swelling and shrinking characteristics. - Dispersion and flocculation- As long as the colloidal particles remain negatively charged, they repel each other and the suspension remains stable. If the magnitude of the charge is reduced, the particles coalesce, form flock or loose aggregates, and settle down. The process of coalescence and formation of flocks is known as flocculation.
De-flocculation or dispersion is the breaking up of flocks into individual particles. It is opposite of flocculation. - Brownian movement- The oscillation is due to the collision of colloidal particles with those of the liquid in which they are suspended. Soil colloidal particles with those of water in which they are suspended are always in a constant state of motion. The smaller the particle, the more rapid is its movement.
- Non permeability- Unlike crystalloids, colloids is unable to pass through a semi-permeable membrane. Even though the colloidal particles are extremely small, they are bigger than molecules of crystalloid dissolved in water.
Types of Soil Colloids
There are four major types of colloids present in soil.
- Layer silicate clays
- Iron and aluminum oxide clays (sesquioxide clays)
- Allophane and associated amorphous clays
- Humus Layer silicate clays
Iron and aluminum oxide clays, allophane and associated amorphous clays are inorganic colloids while humus is an organic colloid.
Layer silicate clays
- Also known as phyllosilicates because of their leaf-like or plate like structure (Phyllon means leaf).
- These are made up of two kinds of horizontal sheets. One dominated by silicon and other by aluminum and/or magnesium.
Silica tetrahedron: It is called the silica tetrahedron because one silicon atom is surrounded by four oxygen atoms. An interlocking array or a series of these silica tetrahedra tied together horizontally by shared oxygen anions gives a tetrahedral sheet.
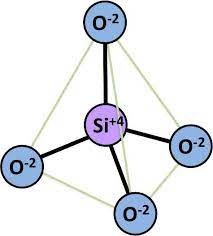
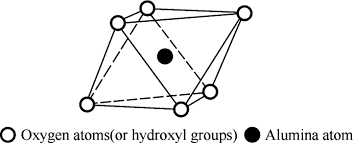
Alumina octahedron: Aluminum and/or magnesium ions are the key cations surrounded by six oxygen atoms or hydroxyl group giving an eight sided building block termed as octahedron. An aluminum-dominated sheet is known as a di-octahedral sheet, whereas magnesium dominated sheet is called a tri-octahedral sheet. The distinction is due to the fact that two aluminum ions in a di-octahedral sheet satisfy the same negative charge from surrounding oxygen and hydroxyls as three magnesium ions in a tri-octahedral sheet. The tetrahedral and octahedral sheets are the fundamental structural units of silicate clays.
Iron and aluminum oxide clays (sesquioxide clays)
- A sesquioxide is an oxide containing three atoms of oxygen with two atoms of another element.
- Under extensive leaching by rainfall and prolonged intensive weathering of minerals in humid warm climates, most of the silica and alumina in primary minerals are dissolved and slowly leached away. The remaining materials which have lower solubility are called sesquioxides.
- Sesquioxides are mixtures of aluminum hydroxide, Al (OH)3, and iron oxide (Fe2O3), or iron hydroxide, Fe(OH)3. These clays can grade from amorphous to crystalline. Examples of iron and aluminum oxides common in soils are gibbsite (Al2O3.3H2O) and geothite (Fe2O3.H2O).
- These clays do not swell, not sticky and have high phosphorus adsorption capacity.
Allophane and other amorphous minerals
- These silicate clays are mixtures of silica and alumina.
- They are amorphous in nature.
- These clays occur where large amount of weathered products existed.
- These clays are common in soils forming from volcanic ash (e.g., Allophane).
- These clays have high anion exchange capacity or even high cation exchange capacity. Almost all of their charge is from accessible hydroxyl ions (OH-), which can attract a positive ion or lose the H+ attached.
Humus (Organic Colloid)
- It is a temporary intermediate product (continue to decompose) left after considerable decomposition of plant and animal remains.
- Humus is amorphous, dark brown to black, nearly insoluble in water, but mostly soluble in dilute alkali (NaOH or KOH) solutions.
- The humus colloids are not crystalline. They are composed basically of carbon, hydrogen, and oxygen rather than of silicon, aluminum, iron, oxygen, and hydroxyl groups.
- The organic colloidal particles vary in size, but they may be as small as the silicate clay particles. The negative charges of humus are associated with partially dissociated (-OH), carboxyl (-COOH), and phenolic groups; these groups in turn are associated with central units of varying size and complexity.
Difference between organic and inorganic colloids
Humus Clay Made up of C,H,O Made up of Si, Ai, O Complex amorphous organic colloid Inorganic and crystalline More dynamic, formed and destroyed more rapidly Relatively stable Complex structure not well known Clays have definite and well known structure
Importance of soil colloids
- Soil colloids are the most active constituent of the soil and it determine the physical and chemical properties of the soil.
- The colloidal surface attracts soil nutrients dissolved in soil, water as positively charged mineral ions or cations.
- Some cations like calcium (Ca++), Potassium (K+), and sodium (Na+) are needed for plant growth. They need to be dissolved in a soil-water solution to be available for plants.
- Without soil colloids, most vital nutrients would be leached out the soil by percolating water and carried away in streams.
- A high base status means that the soil holds an abundant supply of base cations necessary for plant growth. If soil colloids hold a small supply of bases, the soil is of low base status and is, therefore, less fertile.
- Humus colloids have high soil fertility.
Frequently Asked Question
What is the size of soil colloids?
Particles less than 0.001 mm diameter in size possess colloidal properties known as soil colloids.
What is zeta potential?
It refers to the electrical charge at the surface of the hydrodynamic shear surrounding the colloidal particles.
What do you mean by cohesion and Adhesion?
Cohesion refers to the tendency of clay particles to stick together; whereas adhesion refers to the attraction of colloidal materials to the surface of any other substance with which it comes in contact.
What is Brownian movement?
Brownian motion is the random motion of a particle as a result of collisions with surrounding gaseous molecules.
What is sesquioxide clay?
A sesquioxide is an oxide containing three atoms of oxygen with two atoms of another element. Sesquioxides are mixtures of aluminum hydroxide, Al (OH)3, and iron oxide (Fe2O3), or iron hydroxide, Fe(OH)3.
Examples of iron and aluminum oxides common in soils are gibbsite (Al2O3.3H2O) and geothite (Fe2O3.H2O). These clays do not swell, not sticky and have high phosphorus adsorption capacity.